Chronic Pain Clinic
Current Methodology
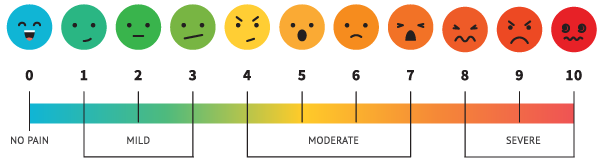
To gauge a patient’s pain state physicians rely on a set of questions which are answered by the patient, the most common being the numeric rating scale (NRS). The NRS requires patients to evaluate their own pain level and communicate that rating on a scale from 0-10 (0= no pain: 10= worst pain imaginable).
Once a baseline is determined the physician determines the most appropriate treatment taking into account the patient’s reported pain state and medical history. Follow-up visits are required to determine whether treatment has led to an acceptable reduction of a patient’s pain state from baseline.
Current Standard of Care: Numeric Rating Scale (NRS)
Not Verifiable
Not Replicable
Subjective
Problems with Current Methodology:
Patients may have difficulties providing a good estimate of their pain state. This problem is compounded by the fact that patients have no good basis for differentiating pain scores, especially in the middle of the scale (3-7); they can only compare to memories or imagined pain. Problems also exist for patients who have difficulty communicating, such as Alzheimer’s patients, or who intentionally mis-communicate their pain in order to obtain drugs or remain on disability.
Problems with Current Methodology Lead to:
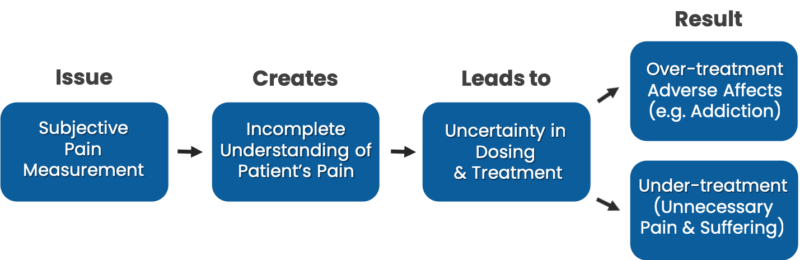
Lack of a reliable baseline creates uncertainty in the selection of initial pain therapy. Subjective pain scoring variability from visit to visit also creates uncertainty whether treatment is working. This uncertainty leads to unnecessary cost to the health care system due to poor patient outcomes. Specific issues include:
- Over-treatment of patients can lead to addiction to opioids.
- Under-treatment of patients can lead to unnecessary pain and multiple trips to the emergency room or other healthcare venue.
- Lack of a reliable, objective patient pain assessment increases physician liability exposure as disputes are focused on patient reported results.
Solution for Chronic Pain Clinics
The PainQx platform system is currently an investigation medical device. The PainQx platform will be seeking FDA Clearance as a Class II medical device in 2020.
The PainQx platform upon FDA Clearance will address the issues illustrated below by providing:
- Objective Pain Measurement: Improving patient outcomes
- Patient Pain State Confirmation: Empowering practitioner’s clinical decisions
- Effective Pain Management: Addresses under / over treatment dilemma
Assessing A Patient’s Pain State with PainQx:
Patients’ brain waves are analyzed and PainQx generates a Pain Classification for the patient. Current classification categories include no pain, mild to moderate pain, and severe pain. The pain categories have been chosen to correspond to different pain treatment protocols such as physical therapy, surgery, over the counter NSAIDS, prescription opioids, etc. PainQx is working to provide additional granularity in the pain categories. The PainQx pain classification is intended to be used as adjunctive information. Once the patient examination is completed, the physician makes a treatment decision based on the patient’s PainQx pain classification, medical history, patient presentation/symptoms, and the self-reported NRS pain score.
The presence of a quantifiable and objective tool that provides information on a patient’s pain state reduces a physician’s liability during disputes as independent, objective information can be included in the discussion
Advantages of PainQx Methodology:
The PainQx system is a quantifiable and objective assessment tool that the doctor can use to establish an initial patient pain state and assess changes in pain classification as part of subsequent visits.
- Reduces reliance on the subjective, patient provided pain score
- Removes sociological influences inherent in collecting subjective, verbal information from the patient regarding their level of pain
- Provides a physiologically based assessment, reducing variability associated with subjective reporting
Advantages of the PainQx Platform include:
- A reliable, objective assessment across visits reduces uncertainty of treatment leading to improved clinical outcomes and savings to the healthcare system
- Improved dosing leads to fewer incidence of opioid abuse thereby reducing the risk of addiction or drug diversion
- Improved dosing leads to a reduction in under-treatment, improving patient satisfaction
Pain Therapy Clinical Research
Current Methodology
To gauge a patient’s pain state, clinical researchers use the numeric rating scale (NRS) which is a scale between 0-10 (0: no pain & 10: worst pain imaginable). Patients are asked to report their pain levels between 0-10 pre and post intervention; if a reduction is shown relative to placebo than the drug is considered efficacious.
Problems with Current Methodology:
Patients have trouble providing a good estimate of their pain state and have no good basis for differentiating between pain states especially in the middle of the scale (3-7).
Problems with Current Methodology Lead to:
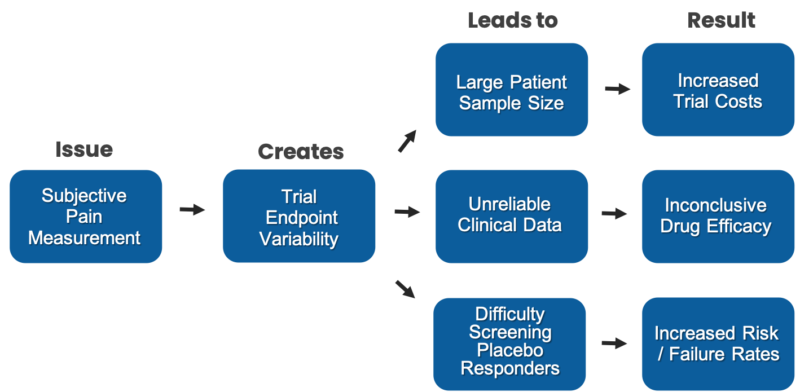
- High failure rate of pain drug trials due to variability in subjective patient pain reporting
- Increased cost of clinical trials caused by the necessity to smooth out variability in patient pain reporting through the use of large patient sample sizes
- Loss of investor interest due to erratic measurement of the clinical study end point
Solution for Pain Therapy Clinical Research
PainQx addresses the challenges illustrated below by providing an Objective Pain Assessment which reduces clinical trial costs and risks.
PainQx Pain classification: Provides objective, physiologically based data, helps quantify research outcomes; validates research products.
Patients’ brain waves are analyzed and PainQx generates a Pain Classification pre and post intervention. Current classification categories include no pain, mild to moderate pain, and severe pain. If a reduction in pain classification is shown relative to placebo than the drug is considered efficacious. It may also be possible to use the discriminant scores generated by the PainQx binary classifiers to create a more granular, continuous pain score for the purpose of Pain Therapy Clinical Study support.
Advantages of PainQx Methodology:
The PQx score is a quantifiable and objective assessment that can be used to evaluate the efficacy of an analgesic drug or other therapy:
- Removes the need for study participants to verbalize their pain state.
- Removes sociological influences inherent in collecting subjective, verbal information from the patient regarding their level of pain.
- Requires no esoteric equipment and can be easily implemented in a clinical study environment.
Advantages of PainQx System include:
- Reduces failure rate of pain drug trials by providing objective, physiologically based patient pain reporting.
- Clinical trial costs are significantly reduced due to the reduction in sample sizes facilitated through a decrease in patient reporting variability.
- Increased investor interest as clinical study end points become less erratic, increasing the likelihood of FDA approval.

